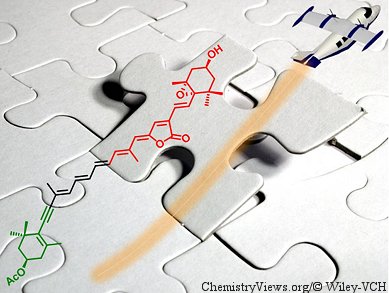
Carotenoids play an important dual role in photosynthetic organisms because they act as antennae that transfer the sun’s energy to chlorophyll and, hence, to the photoreaction centers. They also protect the natural photosystems from damage caused by excess light. Among the wide variety of carotenoids isolated from marine organisms, peridinin and fucoxanthin are the most abundant. Pyrrhoxanthin, isolated from microalgae and planktonic dinoflagellates, is structurally closely related to peridinin; however, the challenges posed by the structures of these molecules have made them difficult targets for total synthesis.
Belén Vaz, Ángel R. de Lera, and co-workers, Universidade de Vigo, Lagoas-Marocosende, Spain, have reported the stereocontrolled total synthesis of enantiopure C37-norcarotenoid pyrrhoxanthin. The last step of this synthesis involved a stereoselective Horner–Wadsworth–Emmons condensation. Different approaches to the construction of the alkylidenebutenolide fragment of pyrrhoxanthin were explored. The resulting regio- and stereoselective silver-promoted lactonization of pentenynoic acids was shown to be robust, as demonstrated by the construction of a range of alkylidenebutyrolactones from pentenynoic acids of increasing complexity.
- Total Synthesis of Enantiopure Pyrrhoxanthin: Alternative Methods for the Stereoselective Preparation of 4-Alkylidenebutenolides,
Belén Vaz, Leticia Otero, Rosana Álvarez, Ángel R. de Lera,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301873