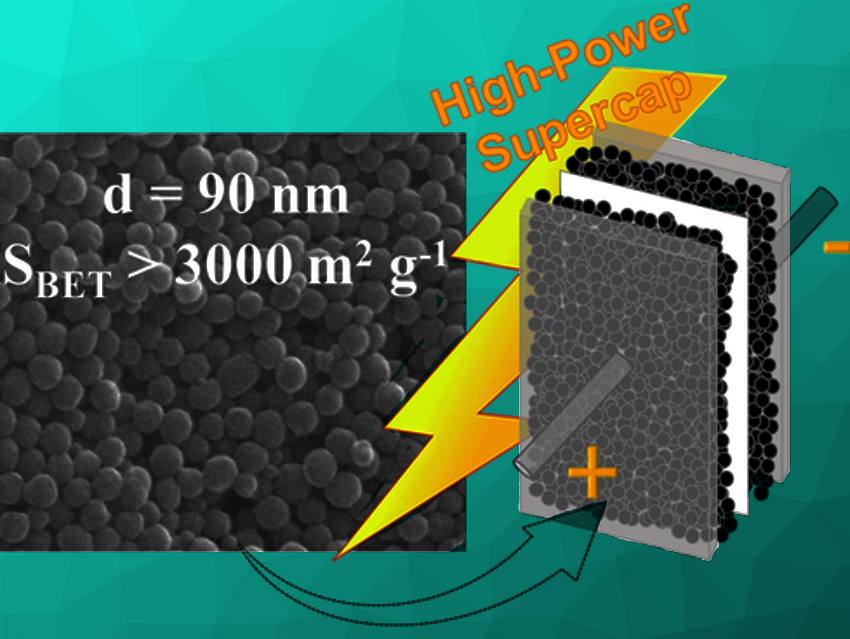
Supercapacitors store and deliver electrical energy in seconds, as opposed to power-limited batteries. Microporous carbons are the typical electrode material used in supercapacitors given their large specific surface area, which makes them able to adsorb a large number of electrolyte ions. Conventional porous carbons with sizes above 10 µm have a tortuous pore structure that offers resistance to ion migration and limits the power performance. Thus, designing highly porous nanosized carbons with readily accessible micropores is a challenge.
Noel Díez, Marta Sevilla, Alba Fombona, and Antonio B. Fuertes, Instituto de Ciencia y Tecnología del Carbono (INCAR-CSIC), Oviedo, Spain, have developed an electrode material for supercapacitors that consists of uniform carbon nanospheres (d = 90 nm) with an ultra-high specific surface area exceeding 3000 m2g–1 and very short pathways for ion diffusion.
The nanospheres were prepared by the oxidative polymerization of pyrrole with FeCl3 as anoxidant, and in the presence of a stabilizer to block the precipitation and coalescence of the generated polymeric particles. The their direct chemical activation with KHCO3 follows. The morphology of the nanospheres is well retained after activation, and the inorganic impurities are removed by a simple washing step with water.

The nanoparticles were tested as electrodes in supercapacitors using mass loadings typical of commercial devices (ca. 10 mg cm−2). The porous nanoparticles achieved a high capacitance and showed an excellent response at high power conditions, even when tested in high voltage organic electrolytes, wherein they achieved a large energy density of 31 Wh kg–1 at a high power density of 31 kW kg–1.
- Monodisperse Porous Carbon Nanospheres with Ultra‐High Surface Area for Energy Storage in Electrochemical Capacitors,
Noel Díez, Marta Sevilla, Alba Fombona‐Pascual, Antonio B. Fuertes,
Batteries & Supercaps 2021.
https://doi.org/10.1002/batt.202100169