There is great interest in methods that can reduce the amount of CO2 in the atmosphere, e.g., carbon capture and storage (CSS). Metal–organic frameworks (MOFs) and zeolites are typically used for this task. However, alternative materials that sequester CO2 have also been investigated.
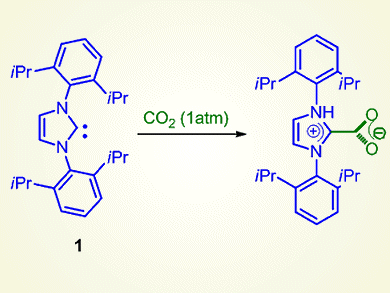
Previously, Janis Louie and co-workers, University of Utah, USA, reported that N-heterocyclic carbene (1) can react reversibly in solution with CO2 to give a zwitterion [1]. Obtaining sorption isotherm data on (1) has proven difficult as the zwitterion is relatively insoluble in solvents with low vapor pressures.
This issue prompted William Schneider, Joan Brennecke, and Brandon Ashfeld, University of Notre Dame, IN, USA, to examine chemisorption properties of (1) as a free-flowing powder with CO2 to investigate N-heterocyclic carbenes as neutral reagents for carbon capture [2]. The group used single crystal X-ray diffraction to examine the structure of (1) before and after CO2 uptake. The group hypothesize that the rapid uptake of CO2 is associated with a facile reorganization of the structure of (1), thus allowing CO2 to enter the solid framework between the layers.
The researchers say their findings will have direct implications on further design strategies for CCS reagents, whether for pre- or post-combustion flue-gas separation from coal or natural-gas-fired fuel power plants.
- A Systematic Investigation of Factors Influencing the Decarboxylation of Imidazolium Carboxylates,
B. R. Van Ausdall, J. L. Glass, K. M. Wiggins, A. M. Aarif, J. Louie,
J. Org. Chem. 2009, 74, 7935–7942.
DOI: 10.1021/jo901791k - Solid State Covalent Capture of CO2 Using N-Heterocyclic Carbenes,
Monika Vogt, Joshua E. Bennett, Yong Huang, Chao Wu, William F. Schneider, Joan F. Brennecke, Brandon L. Ashfeld,
Chem. Eur. J. 2013, 19, 11134–11138.
DOI: 10.1002/chem.201302013



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)