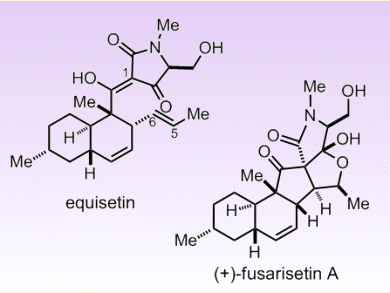
Tetramic acids are an important class of naturally occurring compounds with great medicinal potential. A representative of this group is equisetin, a fungal metabolite, which was discovered from the white mold Fusarium equiseti. Equisetin exhibits potent antibiotic/HIV inhibitory activity and selective cytotoxicity to mammalian cells. Recently, a new member of this group, (+)-fusarisetin A, isolated from soil fungus Fusarium sp. FN080326, has been found to potently inhibit acinar morphogenesis, cell migration, and cell invasion in MDAMB-231 cells without significant cytotoxicity, thus making it a promising anticancer agent.
Shuanhu Gao and co-workers, East China Normal University, China, have accomplished the biomimetic syntheses of equisetin and (+)-fusarisetin A. The synthetic routes, starting from a polyenoylamino acid, were based on the hypothesis that both of these compounds share the same biosynthetic pathway. Key steps include an intramolecular Diels–Alder reaction and a subsequent Dieckmann cyclization of polyenoylamino ester to furnish equisetin. Aerobic oxidation of equisetin mediated by a MnIII/O2 system or a reactive oxygen species gives (+)-fusarisetin A via peroxyfusarisetin.
- Biomimetic Synthesis of Equisetin and (+)-Fusarisetin A
Jun Yin, Lili Kong, Cheng Wang, Yingbo Shi, Shujun Cai, Shuanhu Gao
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302163