The Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam is one of the most important chemical steps in the synthesis of this very important precursor of nylon-6. Generally, oleum, also known as fuming sulfuric acid, is used as catalyst for this rearrangement. Up until now, all published microreactor-type Beckmann rearrangement experiments with solutions of cyclohexanone oxime were performed in the once-through mode and/or internal recirculation was mimicked by adding the final product ε-caprolactam to the oleum.
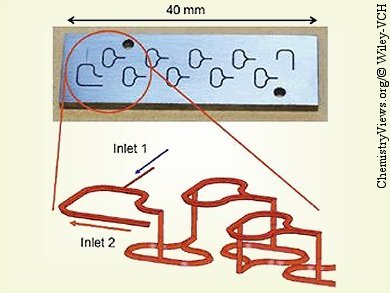
Jaap C. Schouten and co-workers, Eindhoven University of Technology, The Netherlands, experimentally verified the Beckmann rearrangement of a solution of cyclohexanone oxime in cyclooctane with oleum in a microreactor with internal recirculation. The microreactor setup is comprised of combination of micromixers and microchannels. Two split-and-recombine micromixers were used for the mixing of ε-caprolactam in oleum with additional oleum and for the mixing of ε-caprolactam in oleum with solutions of cyclohexanone oxime in cyclooctane.
This method demonstrates the reliability of using microreactors with hazardous chemicals.
- Beckmann Rearrangement of Cyclohexanone Oxime in a Microreactor Setup with Internal Recirculation,
N. T. Zuidhof, M. H. J. M. de Croon, J. C. Schouten, J. T. Tinge,
Chem. Eng. Technol. 2013, 36, 1387–1394.
DOI: 10.1002/ceat.201300088