Multicomponent reactions constitute a fundamental organic chemistry methodology as they embody many features of the ideal synthesis, such as convergence, atom and step economy, synthetic versatility, and selectivity. Nevertheless, understanding the selectivity factors that discriminate among several evolutionary pathways is still challenging. As they are intrinsically complex processes, owing to the coexistence of three or more reactants, few detailed mechanistic studies are known for these reactions.
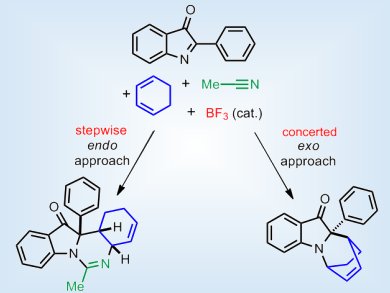
Rodolfo Lavilla, F. Javier Luque, and co-workers, Barcelona Science Park and University of Barcelona, Spain, have conducted a mechanistic and computational analysis of a multicomponent system formed of cyclohexadiene, 2-phenyl-indol-3-one, and acetonitrile. They reveal a divergent reaction mechanism where the relative orientation of the initial reactants dictates the synthetic outcome: The exo approach led to formation of the imino-Diels-Alder product in a concerted manner, and the alternative endo pathway followed a different course to afford the Mannich-Ritter-amidine adduct through a stepwise process. The stereochemical outcome was explained by the rationalization of these results.
This provides a basis to understanding the behavior of these important systems.
- Evolution of a Multicomponent System: Computational and Mechanistic Studies on the Chemo- and Stereoselectivity of a Divergent Process,
Salomé Llabrés, Esther Vicente-García, Sara Preciado, Cristina Guiu, Ramon Pouplana, Rodolfo Lavilla, F. Javier Luque,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302072