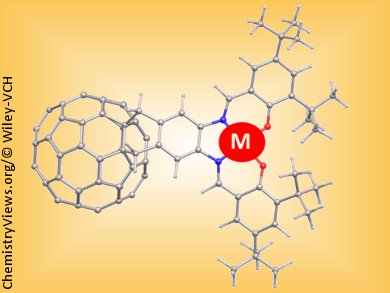
The combination of fullerene bound to transition metal ions can be utilized to form molecular or supramolecular systems with tunable physicochemical properties. Maria Lebedeva and co-workers, under the supervision of Andrei N. Khlobystov and Martin Schröder, University of Nottingham, UK, have developed a five-step synthetic procedure to link the fullerene C60 and salen units to give a new hybrid ligand that can bind a wide range of transition metals.
In the past the major problems in this area of chemistry have been the difficulty of synthesis of the fullerene containing metal-binding systems and also solubility of the ligand and the stability of the resulting metal complexes. These problems have been overcome by the inclusion of tBu groups in the salen part of the ligand.
The presence of the metal–salen group not only affects the optical properties of the fullerene derivatives by inducing strong absorptions in a wider spectral range than the parent fullerene, but also enhances the redox properties of these dyad molecules by the addition of further acceptor orbitals and, more importantly, by the incorporation of readily accessible donor orbitals.
In addition, metal–salen complexes are known as catalysts for a variety of reactions. The group at Nottingham has shown that the fullerene does not have an adverse effect on the catalytic properties. The presence of the fullerene moiety has enabled the researchers to load these complexes onto single-, double- and multiwalled carbon nanotubes and graphitized carbon nanofibres. This allows to construct nanoscale architectures with well-defined structure and functional properties that can be exploited in electronic devices, chemical nanoreactors, and heterogeneous catalysis.
- Transition Metal Complexes of a Salen–Fullerene Dyad: Redox and Catalytically Active Nanostructures for Delivery of Metals in Nanotubes,
M. A. Lebedeva, T. W. Chamberlain, E. S. Davies, D. Mancel, B. E. Thomas, M. Suyetin, E. Bichoutskaia, M. Schröder, A. N. Khlobystov,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300872