Glycopeptides and glycolipids play a crucial role in various biological processes, such as protein structure modulation and cell–cell recognition. Therefore, their use as drugs would be very promising if their efficiency were not undermined by their low metabolic stability, owing to in vivo cleavage of the anomeric bond by glycosidases. The resulting low bioavailability of carbohydrate-based drugs is a severe drawback that could be overcome by the creation of analogues with similar bioactivity and increased in vivo stability. C-glycosides were the first structures to be developed for this purpose
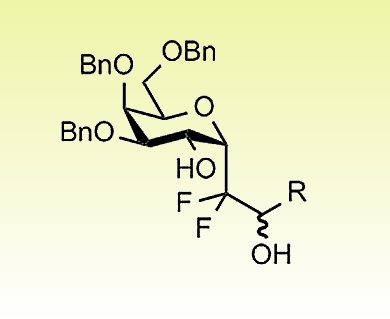
Eric Leclerc, Xavier Pannecoucke, and co-workers, Institut Charles Gerhardt and Normandie Université, France, describe the synthesis of fluorinated α-C-glycosides with complete diastereoselectivity, by the addition of difluoromethyl radicals to 2-benzyloxyglycals, followed by reduction of the resulting 2-ketohexopyranosides. The presence of CF2CO2iPr or CF2Br groups at the pseudo-anomeric position of the α-C-glycosides allows efficient reduction/olefination or Br/Li-exchange/nucleophilic-addition sequences.
This strategy opens the way for the synthesis of fluorinated C-glycosidic analogues of glycoconjugates.
- Addition of Electrophilic Radicals to 2-Benzyloxyglycals: Synthesis and Functionalization of Fluorinated α-C-Glycosides and Derivatives,
Sophie Colombel, Nathalie Van Hijfte, Thomas Poisson, Eric Leclerc, Xavier Pannecoucke,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302070