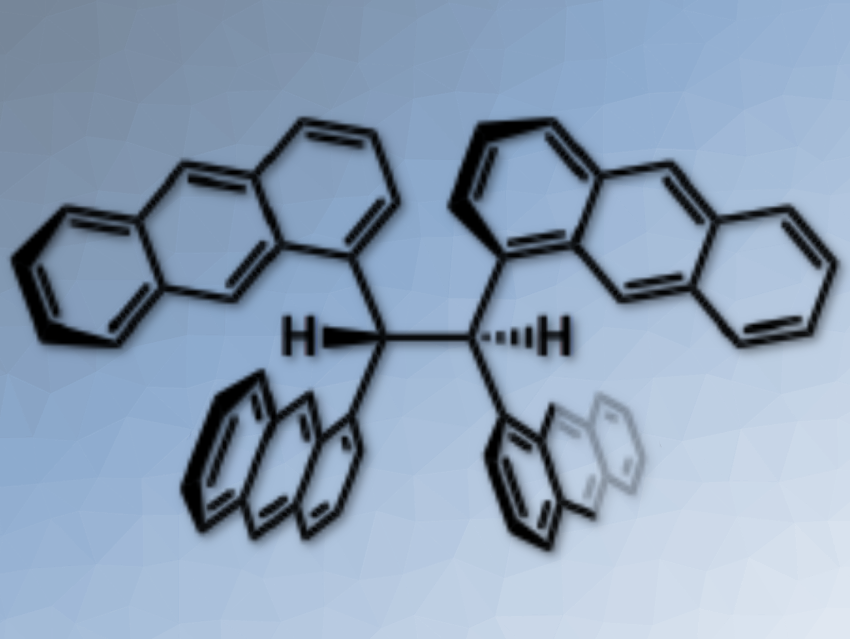
An anthracene is a fascinating building unit to construct novel aromatic hydrocarbons. Its fluorescent properties have been applied to develop emissive materials such as functional dyes and sensing probes. When multiple anthracene units are assembled in a molecule, the photophysical properties are considerably influenced by their relative orientation.
Shinji Toyota, Tokyo Institute of Technology, Japan, and colleagues have synthesized an ethane derivative, in which the C(sp3)–C(sp3) bond was substituted with four 1-anthryl units (pictured). In the stable conformation, two vicinal anthracene units were stacked and the other units extended away without stacking.
To synthesize the compound, the team reacted 1-bromoanthracene (5) with 1-anthracenecarbaldehyde to di(1-anthryl)methanol (7) (pictured below). This alcohol was oxidized with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to a ketone. Finally, a McMurry coupling was conducted using a standard method with TiCl4 and Zn in DME.

In the fluorescence spectrum, this compound showed a characteristic broad band due to excimer-type emission in contrast to other hydrocarbons with two anthracene units. These data indicate that the introduction of four anthracene units into an ethane core is a key factor to regulate conformational preference and tune photophysical properties.
According to the researchers, this molecular design can be applied to the synthesis of novel anthracene assembled compounds having multiple layered units or undergoing photochemical reactions.
- Structure and Photophysical Properties of 1,1,2,2‐Tetra(1‐anthryl)ethane: A C(sp3)–C(sp3) Bond Substituted with Four Anthracene Units,
Shu Aoki, Eiji Tsurumaki, Masahiro Yamashina, Kan Wakamatsu, Shinji Toyota,
ChemPlusChem 2021.
https://doi.org/10.1002/cplu.202100447