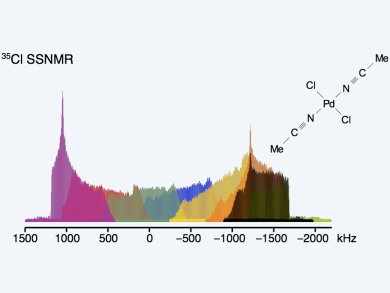
Characterization of the molecular-level structures of heterogeneous catalysts is important for understanding how they work. There are many chlorine-containing transition-metal organometallic and inorganic catalysts in use today in a wide range of organic reactions and polymerization processes. Robert W. Schurko and his team, University of Windsor, Canada, in collaboration with groups from the Université Lille Nord de France, France, the Steacie Institute for Molecular Sciences, Canada, and ESCPE Lyon, France, have combined 35Cl solid-state NMR (SSNMR) and 35Cl nuclear quadrupole resonance (NQR) spectroscopies and DFT calculations of NMR interaction tensors for the structural characterization of chlorine-containing complexes.
The use of a special pulse sequence allows for the quick acquisition of 35Cl SSNMR spectra at both moderate and high magnetic fields and can distinguish between bridging and terminal chlorine environments on the basis of the quadrupolar parameters. 35Cl SSNMR and NQR spectroscopies are themselves complementary techniques: the former provides approximate values of quadrupolar coupling constants (CQ) and asymmetry parameters (ηQ), whereas NQR can be used to identify multiple sites with similar quadrupolar parameters and to refine values of CQ. First-principles 35Cl DFT calculations provide important information on the relationship between the 35Cl electric field gradient (EFG) tensors and molecular structure. Additionally, for the first time, 35Cl SSNMR is used to observe TiCl4 supported on mesoporous silica.
This combination of techniques is a promising and simple method for the characterization of all manner of chlorine-containing transition-metal complexes, in both bulk and supported forms.
- A Study of Transition-Metal Organometallic Complexes Combining 35Cl Solid-State NMR and 35Cl NQR Spectroscopy and First-Principles DFT Calculations,
K. E. Johnston, C. A. O’Keefe, R. M. Gauvin, J. Trébosc, L. Delevoye, J.-P. Amoureux, N. Popoff, M. Taoufik, K. Oudatchin, R. W. Schurko,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301268