Bismuth compounds, such as bismuth subsalicylate, are of medical importance. In addition, bismuth oxido clusters represent prominent molecular intermediates to bismuth oxide polymorphs that offer potential in material science, such as ionic conductors and photocatalysts.
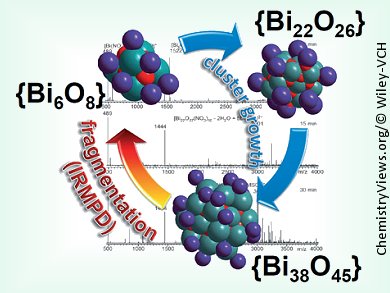
Christoph Schalley, Freie Universität Berlin, Germany, Michael Mehring, Technische Universität Chemnitz, Germany, and co-workers have applied tandem mass spectrometric experiments to bismuth–oxido clusters to complement solid-state and solution studies and enhance the understanding of their reactivity. Previously, this technique has been only rarely applied to such nanosized clusters. The team found that cluster growth ocurred from {Bi6O8} to {Bi22O27} and finally {Bi38O45} clusters. Also, the reactivity of the organic ligand shell and the cluster core were monitored under environment-free conditions in the high vacuum of the mass spectrometer. For the clusters with an organic ligand shell surrounding the cluster core, a number of different fragmentation processes occur within the ligand shell. In these cases, no fragmentation of the cluster core is observed, thus pointing to its high stability.
These gas-phase experiments open a new view on the clusters’ intrinsic reactivity, which significantly deviates from that in solution.
- Mass Spectrometry and Gas-Phase Chemistry of Bismuth–Oxido Clusters,
Dominik Sattler, Maik Schlesinger, Michael Mehring, Christoph A. Schalley,
ChemPlusChem 2013.
DOI: 10.1002/cplu.201300122