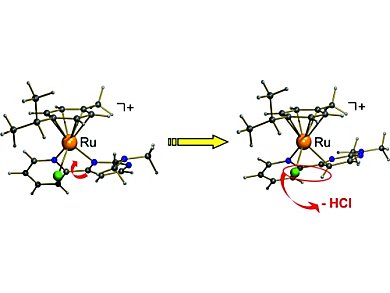
For the transfer hydrogenation of ketones, ruthenium(II) complexes have been the most active systems. To activate the pre-catalyst, the presence of a base seemed to be unavoidable.
Gereon Niedner-Schatteburg, Werner R. Thiel, and colleagues, Technische Universität Kaiserslautern, Germany, found that phosphane-free and air-stable transfer-hydrogenation complexes catalyze the transfer hydrogenation of acetophenone in the absence of a base. Slight variation of the functional group attached to a nitrogen donor ligand lead to this first phosphane-free and air-stable transfer-hydrogenation catalyst. The key step in the catalyst self-activation process is a C–H bond cleavage at the pyrimidine part of the bidentate nitrogen donor ligand. This switches from a N,N- to a C,N-coordination mode.
To prove the general applicability of these C–H activation processes in catalysis, the authors are presently examining other transition-metal-catalyzed reactions with catalysts bearing aminopyrimidine based ligands.
- C–H Activation at a Ruthenium(II) Complex – The Key Step for a Base-Free Catalytic Transfer Hydrogenation?,
Leila Taghizadeh Ghoochany, Christian Kerner, Saeid Farsadpour, Fabian Menges, Yu Sun, Gereon Niedner-Schatteburg, Werner R. Thiel,
Eur. J. Inorg. Chem. 2013.
DOI: 10.1002/ejic.201300480