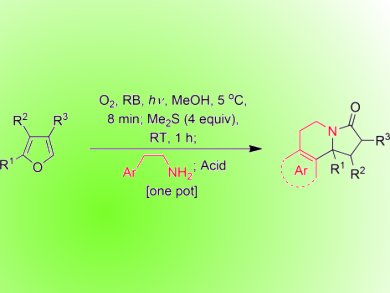
Georgios Vassilkogiannakis and co-workers from the University of Crete, Greece, have reported an interesting method for the formation nitrogen-bearing polycycles. The approach involves photooxidation of furan derivative 1 to generate a 1,4-dielectrophile 2 which reacts with an amine nucleophile to give pyrolidinone 3. Addition of a Brønsted or Lewis acid generates N-acyl iminium ion 4, which undergoes a Pictet–Spengler-type reaction with the pendent aromatic group on the amine to give the final product (see scheme; a more detailed description of the proposed mechanism is given on the first page of the manuscript).

The final Pictet–Spengler step could be achieved with a variety of pendent aromatics although unactivated groups (such as 8) required the use of a stronger Lewis acid (AlCl3). In addition, Mono-, di, and tri-substituted furans were also tolerated, but only activated aromatics would undergo the final cyclization step in the case of the di- and tri-substituted examples.

The reactions are performed in one-pot by sequential addition of reagents and lead to a rapid increase in chemical complexity. Additionally, as many of the products possess the core structure of the erythrina alkaloids this may become a useful synthetic method
- From Simple Furans to Complex Nitrogen-Bearing Aromatic Polycycles by Means of a Flexible and General Reaction Sequence Initiated by Singlet Oxygen,
Dr. Dimitris Kalaitzakis, Dr. Tamsyn Montagnon, Eirini Antonatou, Nuria Bardají, Prof. Dr. Georgios Vassilikogiannakis
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301571