Unusually Bright, Fast Reacting, Biocompatible Probe System
American researchers have developed a probe for marking biomolecules that begins to fluoresce only when it is “switched on” by binding. As reported in the journal Angewandte Chemie, the reaction takes place very quickly and the difference in brightness between the “on” and “off” states is two orders of magnitude bigger than for conventional activatable probes.
Marking biomolecules in living cells with fluorescent probes is a well-established technique. New research possibilities open up when these probes are combined with bioorthogonal reactions. Such reactions can occur inside a living system without disrupting normal biochemical processes. This makes it possible to generate “turn-on” probes: a bioorthogonal reaction binding partner is bound to the biomolecule of interest (without affecting it) and acts as an anchoring site for the fluorescent probe. The probe is devised so that its fluorescence is significantly increased when it binds to the anchoring site. Because the probes not bound to the target fluoresce far less, background fluorescence is reduced. This eliminates the need for complex washing procedures that delay observation of the cells.
For all of this to work, the probe system must work without a toxic catalyst, react quickly to allow for time-resolved observation of biological processes, and fluoresce very strongly after being “turned on” to maximize the signal–strength relative to the background. It has not previously been possible to meet all of these requirements in one system. A team led by Ralph Weissleder at Massachusetts General Hospital and Harvard University, USA, has now developed a system that fits the bill: an unusually bright, fast reacting, biocompatible probe system with a large difference between the switched on and switched off states.
Coupling BODIPY with Tetrazine
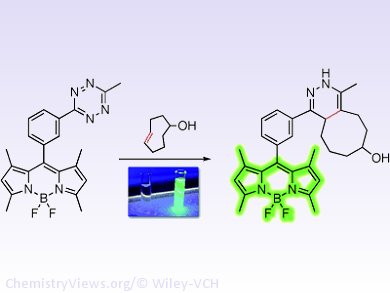
The new probe consists of two components: The first is a fluorescent dye called BODIPY (boron dipyrromethene), a three-ring system with a subunit made of one boron, two nitrogen, and two fluorine atoms. The second component is a tetrazine molecule, a six-membered ring containing four nitrogen and two carbon atoms. Tetrazine quenches the fluorescence of BODIPY, which passes incoming energy off to the tetrazine component without radiation instead of fluorescing. Tetrazine simultaneously serves as a reagent for the bioorthogonal reaction.
The reaction partner is modified trans-cyclooctene (TCO), which the researchers couple to the biomolecule to be studied by means of an antibody. When the probe is added, the tetrazine binds to the TCO, giving off nitrogen and binding the probe to the biomolecule. The reaction destroys the probe’s tetrazine group, turning off the quenching of the fluorescence and allowing the BODIPY molecule to glow an intense green. The researchers recorded fluorescence over a thousand times stronger than that of the probe in the “off” state. This is two orders of magnitude stronger than all previously described turn-on probes.
The success of this system is due to the particularly strong fluorescence quenching made possible by the special electronic constellation and spatial arrangement of the BODIPY and tetrazine components relative to each other.
- BODIPY—Tetrazine Derivatives as Superbright Bioorthogonal Turn-on Probes,
Jonathan C. T. Carlson, Labros G. Meimetis, Scott A. Hilderbrand, Ralph Weissleder,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201301100