Loading a lithium-ion battery produces lithium atoms that are taken up by the graphite layers of the negative electrode. The capacity of graphite is limited to one lithium atom per six carbon atoms. Silicon could take up to ten times more lithium. But, unfortunately, it strongly expands during this process which leads to unsolved problems in battery applications.
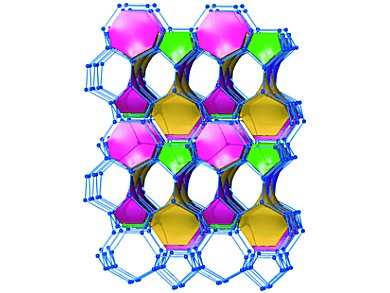
Ulrich Häussermann, Thomas F. Fässler, and colleagues, Technische Universität München (TUM), Germany, have synthesized a framework structure consisting of boron and silicon, which could serve as electrode material. Similar to the carbon atoms in diamond, the boron and silicon atoms in the lithium borosilicide LiBSi2 are interconnected tetrahedrally. But unlike in diamond they form channels.
LiBSi2 is the first example where B and Si atoms form an ordered common framework structure with B engaged exclusively in heteronuclear B–Si contacts. The B–Si net hosts Li atoms in the channels. Thomas Fässler and Michael Zeilinger named their new framework in honor of their university “tum.”
The novel open tetrahedral framework structure (OTF) of the Zintl phase LiBSi2 was made by applying high pressure to a mixture of LiB and elemental silicon. Lithium borosilicide is stable to air and moisture and withstands temperatures up to 800 °C.
- LiBSi2: A Tetrahedral Semiconductor Framework from Boron and Silicon Atoms Bearing Lithium Atoms in the Channels,
Michael Zeilinger, Leo van Wüllen, Daryn Benson, Verina F. Kranak, Sumit Konar, Thomas F. Fässler, Ulrich Häussermann,
Angew. Chem. Int. Ed. 2013, 52 (23), 5978–5982.
DOI: 10.1002/anie.201301540. - Single crystal growth and thermodynamic stability of Li17Si4,
Michael Zeilinger, Daryn Benson, Ulrich Häussermann, Thomas F. Fässler,
Chem. Mat. 2013, 25 (9), 1960–1967.
DOI: 10.1021/cm400612k