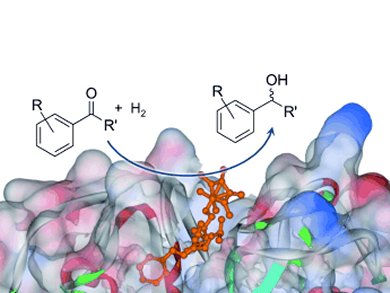
Artificial metalloenzymes could greatly expand the range of reactions accessible by biocatalysis. So far, the most successful strategy to achieve good catalytic activities and enantioselectivities has been through site-directed anchoring of artificial cofactors representing appropriate ligands or metal complexes.
Jörg Eppinger and co-workers, King Abdullah University of Science and Technology, Saudi Arabia, designed modular metal-conjugated affinity labels. They consist of three units:
- an achiral, catalytically active metal complex,
- a protease-specific reactive group that is able to form a covalent bond with the active center of the enzyme, and
- a peptidic affinity tail to direct the binding orientation.
The team showed that variation of the metal center, affinity tail, and the host protein influence the enantioselectivities of a ketone hydrogenation and achieve enantiomeric ratios of up to 82:18.
According to the researchers, this modular setup enables a rapid generation of artificial metalloenzyme libraries, which can be adapted to a broad range of catalytic conditions.
 Metal-Conjugated Affinity Labels: A New Concept to Create Enantioselective Artificial Metalloenzymes,
Metal-Conjugated Affinity Labels: A New Concept to Create Enantioselective Artificial Metalloenzymes,
Thomas Reiner, Dominik Jantke, Alexander N. Marziale, Andreas Raba, Jörg Eppinger,
ChemistryOpen 2013.
DOI: 10.1002/open.201200044