Reactive oxygen species (ROS) and other free radicals can cause damage to biomolecules present in cells, which can ultimately lead to diseases including cancer. A key cellular defense mechanism is the use of antioxidants, such as glutathione. While thio-based radicals have been studied in solution, there are competing reaction pathways present in the solution phase that can be minizimed or eliminated in gas-phase studies, leading to more accurate results.
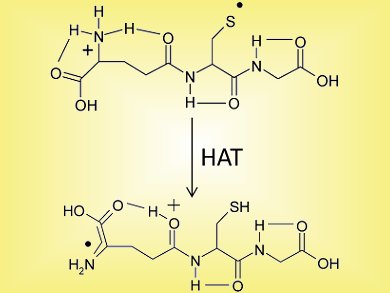
An international collaboration involving groups from Northern Illinois University, University of Florida, both USA, University of Melbourne, Australia, and the FELIX facility, the Netherlands, has used a combination of theoretical and gas-phase experimental methods to examine radical migration in the radical cation of glutathione. Multistage mass spectrometry experiments were used to regiospecifically generate the sulfur-based radical. Ion-molecule reactions that distinguish between sulfur- and α-carbon-based radicals revealed that a slow S to α-carbon radical migration occurs (see scheme).

Infrared multiple-photon dissociation spectroscopy data (shown in red) displayed both the initial sulfur radical and the emerging α-glutamic carbon radicals (peaks at 3425 and 3530 cm–1) in the ion population. Results were confirmed by DFT calculations.
- Structure and Reactivity of the Glutathione Radical Cation: Radical Rearrangement from the Cysteine Sulfur to the Glutamic Acid α-Carbon Atom,
Sandra Osburn, Giel Berden, Jos Oomens, Kerim Gulyuz, Nick C. Polfer, Richard A. J. O’Hair, Victor Ryzhov,
ChemPlusChem 2013.
DOI: 10.1002/cplu.201300057