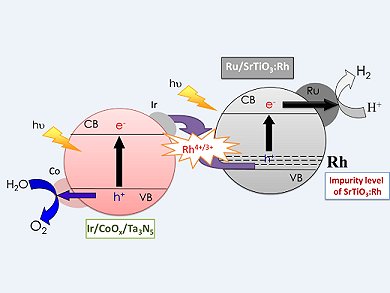
Photocatalytic water splitting is a promising approach to direct conversion of solar energy into H2 and O2. As water and sunlight are freely available, this would lead to a sustainable and renewable energy source. To this end Kazunari Domen, University of Tokyo, Japan, and his collaborators have been investigating the construction of a Z-scheme water-splitting system consisting of Ru/SrTiO3:Rh and Ta3N5 without a redox mediator.
A Z-scheme photocatalysis system usually consists of a two-step photoexcitation system with two photocatalysts—one producing H2 (in this case Ru/SrTiO3:Rh) and the other O2 (in this case modified Ta3N5) that mimics natural photosynthesis. Initially they were able to show that well-crystallized Ta3N5 contributed to efficient water oxidation. Modification of Ta3N5 with nanoparticulate CoOx enhanced the water-oxidation activity, but also suppressed the N2 evolution. Finally, further modification of the O2 catalyst with metallic Ir, which acts as an electron sink, lead to a redox-mediator-free Z-scheme water-splitting system capable of working under simulated sunlight.
- A Redox-Mediator-Free Solar-Driven Z-Scheme Water-Splitting System Consisting of Modified Ta3N5 as Oxygen-Evolution Photocatalyst,
S. S. K. Ma, K. Maeda, T. Hisatomi, M. Tabata, A. Kudo, K. Domen,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300579