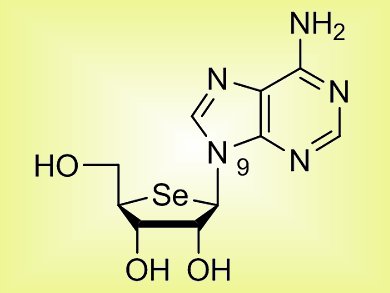
DNA or RNA building blocks have been extensively utilized as excellent templates for the development of modified nucleosides. To develop new therapeutic or biochemical probes, Lak Shin Jeong and co-workers, Ewha Womans University, Seoul, Korea, have turned their attention to next generation 4’-selenonucleosides analogues, which are bioisosterically related to the 4’-oxo- or 4’-thionucleosides (pictured). They highlight the first synthesis and consequent structure revision of 4′-selenoadenosine, starting from D-ribose. Under identical conditions, they have successfully synthesized 4′-selenoguanosine which can also be of significant interest for biological evaluation.

During the synthesis of 1a (pictured), the authors confirmed the rearrangement of the N7– to the N9-isomer, with the aid of X-ray crystal structures.
This study is of great significance as the long standing controversy on the possible regioisomeric rearrangement can be put to rest, at least in the case of 4′-selenopurine nucleosides. The differences in the sugar puckering of the N7– and N9-regioisomers can provide better insights towards the inherent biological activities. Moreover, the X-ray crystal structure of 4′-selenoadenosine revealed an unusual mixture of N and S conformers through A-A base pairing and π-π stacking interactions.
- New RNA Purine Building Blocks, 4′-Selenopurine Nucleosides: First Synthesis and Unusual Mixture of Sugar Puckerings,
Jinha Yu, Jin-Hee Kim, Hyuk Woo Lee, Varughese Alexander, Hee-Chul Ahn, Won Jun Choi, Jungwon Choi, Lak Shin Jeong,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300741