A nice surprise is never a bad thing, and that is exactly what Daniel Gryko and co-workers from the Polish Academy of Sciences in Warsaw recently got in their studies on the Debus-Radziszewski reaction.
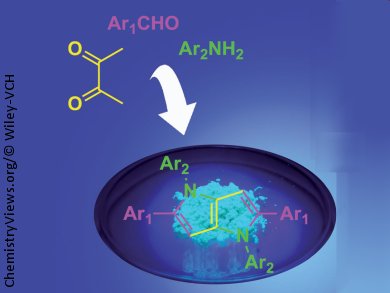
The Debus-Radziszewski reaction is commonly used to produce imidazole derivatives from a mixture of a 1,2-diketone, an aldehyde, and a primary amine. The team was hoping to make new compounds for further work on new functional dyes, but got their wish in a slightly unexpected way. They were surprised to find out that instead of 4,5-dimethylimidazole derivatives, their reactions were forming six bonds at once to give 1,4-dihydropyrrolo[3,2-b]pyrroles. Better still, the products required no chromatography for purification, just crystallisation.
Although this class of compounds is not unknown, the researchers expect that the ability to expediently introduce a wide variety of substituents will lead to more practical applications in the future.
- Synthesis and Optical Properties of Tetraaryl-1,4-dihydropyrrolo[3,2-b]pyrroles,
Anita Janiga, Eliza Glodkowska-Mrowka, Tomasz Stoklosa, Daniel T. Gryko,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201200201