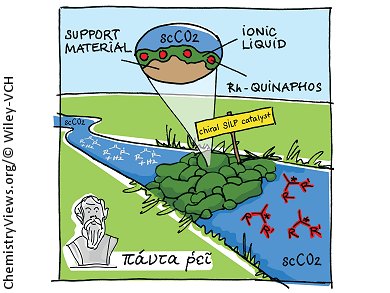
Selectivity, product purity, production efficiency, and flexibility are some important factors for the production of chiral fine chemicals and pharmaceuticals. Walter Leitner and his group in Aachen, Germany, have shown that a combination of a supported ionic-liquid phase (SILP) with supercritical fluids (SFCs) integrated into a continuous-flow process for asymmetric catalysis is a viable option for the production of such compounds.
They followed an integrated approach of concerted development of a catalytic reaction system (molecular scale), the product separation strategy (meso-scale), and the overall process scheme (macro-scale). The asymmetric hydrogenation of dimethylitaconate to dimethyl-2-methylsuccinate was chosen as a model reaction, as enantiopure 2-alkysuccinates are useful building blocks for peptidomimetics, polyesters, and chiral drugs. Essentially chemically and optically pure products were obtained with the catalyst system QUINAPHOS–rhodium, with [EMIM][NTf2], as the ionic liquid, and super critical CO2. This system also reached turnover numbers over 100,000 with space-time yields of 0.7 kg/Lh.
- A Fully Integrated, Continuous-Flow System for Asymmetric Catalysis: Enantioselective Hydrogenation with Supported Ionic Liquid Phase Catalysts by using scCO2 as the Mobile Phase,
U. Hintermair, G. Franciò, W. Leitner
Chem. Eur. J. 2013, 19.
DOI: 10.1002/chem.201204159