Multicomponent reactions (MCRs) are synthetically highly efficient and economical as they offer high structural diversity and incorporate molecular units of all reaction components in a single step. A well-known example is the Mannich reaction. The educts formaldehyde, an amine, and a ketone or aldehyde are converted directly into the adduct.
Compounds with a monofluoromethyl moiety are of great importance for isostere-based drug design. Direct Mannich reactions of fluorinated molecules involving three components are scarce, because often the harsh reaction conditions and the long reaction times lead to unwanted side reactions and products.
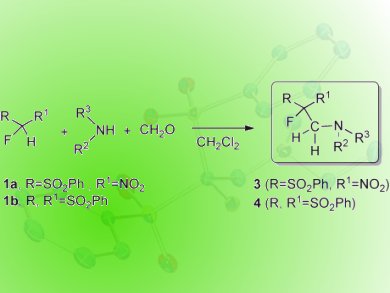
G. K. Surya Prakash, Thomas Mathew, and colleagues, University of Southern California, Los Angeles, CA, USA, report a new protocol (pictured) for the synthesis of fluoroethylated amines by the Mannich-type three-component reaction of formalin, an amine (primary and secondary) and an activated fluoromethane, namely α-fluoro-α-nitro(phenylsulfonyl)-methane (FNSM) or α-fluorobis(phenylsulfonyl)methane (FBSM). By introducing a proper fluoromethyl pronucleophile, the method avoids the use of corrosive or expensive fluorinating reagents such as diethylaminosulfur trifluoride (DAST), morpholinosulfur trifluoride (Morpho-DAST), SF4, and HFamine complexes for selective fluorination.
The products are obtained in high yields and selectivity even, in many cases, without the use of base. The reaction is highly feasible for both primary and secondary amines.
These β-fluoro(phenylsulfonyl)ethylamines may be candidates for further studies on their biological effects and therapeutic activities.
- Direct Synthesis of Diverse β-Fluoroethylamines by a Multicomponent Protocol,
G. K. Surya Prakash, Laxman Gurung, Parag V. Jog, Shinji Tanaka, Tisa Elizabeth Thomas, Nimisha Ganesh, Ralf Haiges, Thomas Mathew, George A. Olah,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201204621