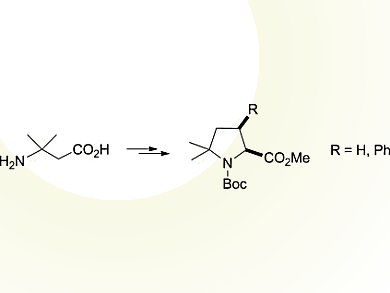
Carlos Cativielaand colleagues, CSIC – Universidad de Zaragoza, Spain, developed a versatile and simple methodology for the preparation of racemic δ,δ-dimethylproline derivatives.
Methyl N-Boc-δ,δ-dimethylprolinate was synthesized from a β-amino acid in six steps and 55 % overall yield. The procedure involves the construction of the pyrrolidine ring through an intramolecular cyclization reaction to form the N–Cα bond.
The methodology can adequately be extended to the preparation of other δ,δ-disubstituted prolines by using different starting β-amino acids.
In addition, it provides access to valuable intermediates for the preparation of δ,δ-dimethylproline derivatives that are functionalized at the β-carbon atom, in particular, the cis stereoisomer of methyl N-Boc-δ,δ-dimethyl-β-phenylprolinate. This has been achieved by coupling phenylboronic acid with a regioselectively generated vinyl triflate followed by a stereoselective hydrogenation. Such δ,δ-disubstituted prolines that are functionalized at the β-position combine the ability to stabilize the cis geometry of the prolyl amide bond with the presence of additional side-chain functionality.
δ,δ-Dimethylproline is being used as a probe to explore the threedimensional structure and folding pathways of therapeutic proteins, the mechanism of important receptors in neuroscience, and the bioactive conformations of peptides.
- Synthesis of Racemic δ,δ-Dimethylproline Derivatives,
Isabel Rodríguez, M. Isabel Calaza, Carlos Cativiela,
Eur. J. Org. Chem. 2013.
DOI: 10.1002/ejoc.201201420