Akin to the metal-mediated activation of O2, the reduction of elemental sulfur (commonly Sn rings) with metals can lead to different metal disulfide species bearing the supersulfide (S2–), disulfide (S22–), or bridging sulfide (S2–) ligand. Understanding these step-by-step transformations in the presence of transition metals is interesting because of their importance in catalytic redox processes and biorelevance. Although the chemistry of transition-metal complexes containing dichalcogenide X22– and bridging X2– ligands is established, few examples of a superchalcogenide X2– ligand have been isolated and characterized. Realization of a dichalcogenide radical ligand (X2•3–, “subchalcogenides”) with an X–X σ bond order of 0.5 is an even more ambitious task as X2n– ligands are expected to act as non-innocent redox ligands.
Recently, a striking copper complex with a {Cu3S2}3+ core caused a debate about the interpretation of its electronic structure because, unlike its oxygen congener {Cu3O2}3+ (local C2v symmetry with one shorter Cu–O bond and two unambiguously O2– ions), the {Cu3S2}3+ core adopts local D3h symmetry with a S•••S distance of 2.69 Å. It may be considered to have a weak attractive S•••S interaction in formal accordance with S2•3– (“subsulfide”) character. However, spectroscopic and computational studies verified the difficulty in assigning formal oxidation states to Cu and S in the system, due to the high symmetry of the core, large covalent character of the Cu–S bonds, and significant S,S→Cu(3d) donation.
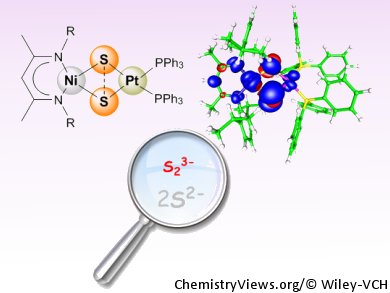
To combat this, Martin Kaupp, Matthias Driess, and co-workers, Technische Universität Berlin, Germany, synthesized a heterobimetallic disulfur monoradical with a diamond-shaped {NiS2Pt} core. Strikingly, the results of DFT calculations and the spectroscopic and structural features revealed that the bonding situation in the S2 ligand is between the elusive ‘half-bonded’ S2 radical trianion (S2•3–) and two separated S2– ligands.
- A Heterobimetallic Approach to Stabilize the Elusive Disulfur Radical Trianion (“Subsulfide”) S2•3–,
S. Yao, P. Hrobárik, F. Meier, R. Rudolph, E. Bill, E. Irran, M. Kaupp, M. Driess,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201203642




In a poly metallic bonding of Sulfur from Hydrogensulfide gas -a situation is obtained where free sulfur separation occurs. This happens in H2S gas scavenging- a situation presented after partial absorption reaction.The reacted sulfur does not regenerate by other reactions. It is difficult to assign bonding locations in pgms+ metal ions.