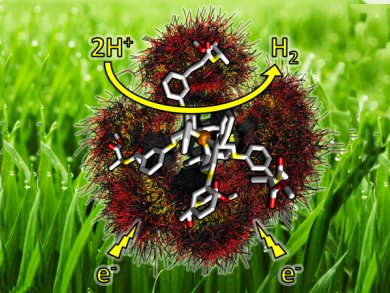
The outer-coordination sphere of metalloenzymes can fine-tune the reactivity and catalytic rates of these complexes. Hydrogenases are a class of enzymes that are gaining significant interest for their ability to efficiently and reversibly reduce H+ to H2 as hydrogen production catalysts.
Simone Raugei, John Roberts, Wendy Shaw, and colleagues at Pacific Northwest National Labs, Richland, USA, and Indiana University of Pennsylvania, USA, present a series of functional hydrogenase mimics and evaluate the effect of an amino acid and dipeptide outer-coordination sphere on the energy efficiency of these complexes. Comparison of the size, aromaticity, and position of these functional groups provides a mechanistic understanding of the role that the environment created by the outer-coordination sphere has on the catalytic activity for hydrogen production.
This environment has been shown to modulate both the rates and overpotentials of hydrogen production; for example, amide, acidic, or basic groups enhance catalysis up to five-fold, and overpotentials were lower with substituents at the N-phenyl meta position. All of the catalysts are active for hydrogen production, with rates averaging ≈1000 s–1, 40 % faster than that of the unmodified catalyst.
- The Role of a Dipeptide Outer-Coordination Sphere on H2-Production Catalysts: Influence on Catalytic Rates and Electron Transfer,
Matthew L. Reback, Bojana Ginovska-Pangovska, Ming-Hsun Ho, Avijita Jain, Thomas C. Squier, Simone Raugei, John A. S. Roberts, Wendy J. Shaw,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201202849