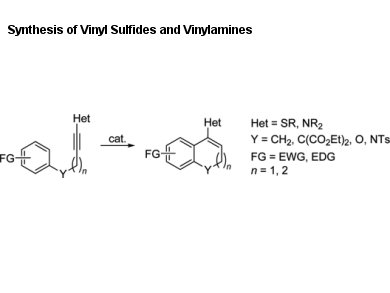
Vinyl sulfides and vinylamines are important building blocks in organic synthesis and are present in many biologically and pharmaceutically active compounds.
Phil Ho Lee, Kangwon National University, Chuncheon, Republic of Korea, and colleagues describe an efficient synthetic method for the preparation of vinyl sulfides and vinylamines through a catalytic intramolecular hydroarylation of arylalkynyl phenyl sulfides and sulfonamides.
Under mild conditions, the catalytic intramolecular hydroarylation reaction was carried out in the presence of FeCl3 and AgOTf (OTf = trifluoromethanesulfonate) in 1,2-dichloroethane. A variety of 1,2-dihydronaphth- alenes, 2H-chromenes, and 1,2-dihydroquinolines containing a phenylsulfenyl or N-phenyl-N-tosyl group on the sp2-hybridized benzylic carbon were prepared in good to excellent yields.
The method could be extended to the preparation of dihydropyrano[2,3-g]chromenes through a twofold Fe-catalyzed hydroarylation by a selective 6-endo mode.
- Synthesis of Vinyl Sulfides and Vinylamines through Catalytic Intramolecular Hydroarylation in the Presence of FeCl3 and AgOTf,
Dahan Eom, Juntae Mo, Phil Ho Lee, Zhiming Gao, Sunggak Kim,
Eur. J. Org. Chem. 2012.
DOI: 10.1002/ejoc.201201270