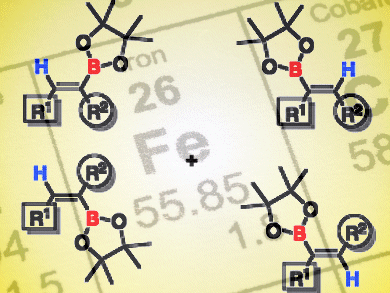
Iron is an attractive metal for catalysis because of its low cost and high abundance. Stephan Enthaler and Michael Haberberger, Technical University Berlin, Germany, used an iron carbonyl complex to catalyze hydroboration of alkynes, that is, the addition of a borane (R2BH) to a carbon–carbon triple bond. The product alkene boranes are useful for further synthetic transformations, for example, in the Suzuki–Miyaura cross-coupling reaction.
Using 2.5 mol % Fe2(CO)9 as catalyst and a reaction time of 24 h in toluene at 100 °C, the authors were able to add pinacolborane to diphenylacetylene with quantitative conversion and high selectivity for the (Z) olefin product. The hydroboration reaction was successful for a variety of substrates, including alkynes bearing substituted aryl, alkyl, and heteroaryl groups. Furthermore, the hydroboration proceeded selectively even in the presence of substrates that are also prone to reduction, such as nitriles and ketones. The reaction can be performed on a scale of 100 mmol under non-inert conditions, thus attesting to its synthetic utility.
- Straightforward Iron-Catalyzed Synthesis of Vinylboronates by the Hydroboration of Alkynes,
Michael Haberberger, Stephan Enthaler,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200931