Conformational Space Characteristics of Biomolecules
Due to their great number of flexible bonds, biomolecules possess a vast conformational space. At ambient temperatures in solution, their free energy landscape has many local minima corresponding to stable conformations that can be easily interconverted by rotations around single bonds.
Gideon J. Davies, University of York, UK, Carme Rovira, University of Barcelona, Spain, and colleagues investigated, through both experimental and theoretical methods, the way in which the GH47 α-mannosidase restricts the conformational space of its substrate, a mannose unit, upon binding. James Naismith, Professor of Chemical Biology at the University of St Andrews, Scotland, UK, emphasizes that this results in “an elegant study of the mechanism of the inverting mannosidase which combines high resolution structural biology and computational chemistry”.
Mannosidases As Vital Biosynthetic Enzymes
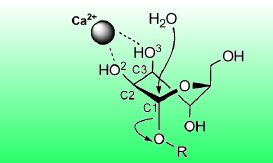
Glycans are poly- or oligosaccharides such as cellulose. In glycoproteins, a glycan is attached to an amino acid residue of a protein. If the glycan is bound to a nitrogen atom of the amino acid residue, it is called an N-glycan. The GH47 α-mannosidase enzyme plays a vital role in the cellular processing and trimming of these N-glycans. As a calcium-dependent α-1,2-mannosidase, it hydrolyzes specific α-1,2-glycosidic linkages between two mannose units in N-glycans and thereby shortens the glycan part of glycoproteins.
A water molecule coordinated by the enzyme acts as the nucleophile and attacks at the anomeric carbon atom of the first mannose unit. Simultaneously, a proton is transferred to the oxygen atom of the α-1,2-glycosidic linkage, turning the second mannose unit into a leaving group (see picture above).
Reduction of The Conformational Space of The Substrate
The six-membered pyran ring of the mannopyranose units of N-glycans exists in solution in a variety of conformations, ranging from chairs and boats to several distorted conformations with skew or half-chair forms. From X-ray structures of the mannosidase with a substrate analogue, the researchers have found that, upon binding to the enzyme, the mannose unit takes on a distinct distorted conformation with a skew form. This is largely supported by the coordination of two hydroxy groups of the mannose unit by the essential calcium ion.
QM/MM calculations and metadynamic simulations provided an explanation as to why this defined conformation is energetically most favorable: calculations of the free energy landscape of the mannose unit with and without the enzymatic environment demonstrated that the enzyme significantly alters the mannose’s free energy landscape and its conformational minima, making the skew conformation the only minimum energy conformation in the enzymatic complex (see Figure 1).
Figure 1: Restriction of the conformational space of a mannose unit by the enzyme and the conformational itinerary. © Wiley-VCH
In the course of the enzymatic reaction catalyzed by the GH47 α-mannosidase, the mannopyranose undergoes continuous conformational change, which is also determined by the enzymatic environment. The researchers showed that the enzyme imposes a narrowing of the free energy landscape of the mannose unit along the reaction coordinate. Owing to interactions with the enzyme, many conformations and conformational interconversions are no longer energetically accessible. Only one low-energy reaction path remains in the free energy landscape of the mannopyranose that connects the reactant, transition state, and product conformations. According to Stephen Withers, Professor of Chemical Biology at the University of British Columbia, Canada, the X-ray structures of exceptionally high resolution, “coupled with the computational studies, provide a high resolution view of the conformational itinerary followed by the enzyme and thus insight into the all-important transition state conformation”.
Designing Enzyme Inhibitors as Transition State Mimics
Highly efficient enzyme inhibitors are often transition state mimics. Hence, knowledge of the transition state and the conformational reaction coordinate provides a scientific basis for the development of inhibitors for GH47 α-mannosidases. “Inhibitors of this enzyme can exquisitely abrogate N-glycan assembly with consequences for a range of diseases associated with N-glycosylation, for example immune diseases”, Stephen Withers explains.
Diversity of Carbohydrate Chemistry
Besides these biomedical aspects, the research results of Davies and his team provide also insight into the diversity of general carbohydrate chemistry and bioorganic reaction mechanisms. Professor David Crich, expert on bioorganic and saccharide chemistry at Wayne State University, Detroit, USA, points out that “studies such as this put our understanding of glycosidase enzymes on an equal footing with that of any other class of enzymes.”
The fact that the nucleophilic attack of the water molecule occurs in what is essentially an SN2-reaction gives proof of the variety of carbohydrate chemistry and makes it interesting to synthetic organic chemists as well: “Contrary to a widely reported statement, carbohydrate chemistry (and biology) is far more than the chemistry of the glycosyl oxocarbenium ion!” Therefore, according to David Crich, the studies “provide a rationale for potential chemical solutions to the problem of stereospecific glycosidic bond formation.”
“This is an outstanding paper that brings to bear synthetic chemistry (inhibitor synthesis), protein crystallography, and computational studies to provide a very high level of insight into the calcium-dependent mechanism of the bacterial GH47 α-mannosidase”, he summarizes the importance of the research results.
- The Reaction Coordinate of a Bacterial GH47 α-Mannosidase: A Combined Quantum Mechanical and Structural Approach,
A. J. Thompson, J. Dabin, J. Iglesias-Fernández, A. Ardèvol, Z. Dinev, S. J. Williams, O. Bande, A. Siriwardena, C. Moreland, T.-C. Hu, D. K. Smith, H.J. Gilbert, C. Rovira, G. J. Davies,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201205338