In biological systems, proteins are the primary means of cellular communication. It relies on a defined spatial orientation of amino acids. However, peptides are not good drug candidates due to their poor bioavailability and limited shelf life. Future lead compounds will likely be nonpeptidic structures. Thus, drug development requires an efficient method of conveying the 3D information in peptide pharmacophores into nonpeptidic structures.
Reverse turns are a common motif in biomolecules, providing many defined 3D structures. Their recognition usually only involves the interaction of amino acid side chains with a receptor, making the peptide backbone unnecessary.
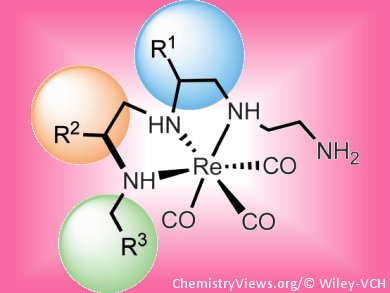
By using a [Re(CO)3]+ center, Jennifer Hickey and Leonard Luyt, University of Western Ontario, Canada, have shown that amino acid sequences can be assembled, reduced, and coordinated on-resin, resulting in Re–ligand reverse-turn mimics. These are structurally distinct from organic molecules due to the variety of geometries and coordination numbers available, potentially providing more chemical entities with an affinity for biological targets.
This method will allow the synthesis of various turn-based peptidomimetics containing a metal scaffold, and aide in the discovery of new drug-like diagnostic and therapeutic radiopharmaceuticals.
- Synthesis of Rhenium-Centric Reverse Turn Mimics,
J. L. Hickey, L. G. Luyt,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201201766