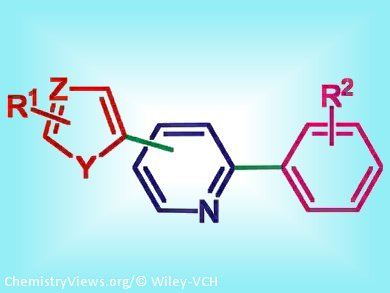
2-Arylpyridine ligands are important building blocks for preparing materials with a variety of applications. For example, the electronic properties of cyclometallated Au, Ir, Pt, or Ru complexes that bear 2-phenylpyridine ligands can be tuned by introducing appropriate substituents, including heteroaryl groups, at various positions. However, the synthesis of such functionalized products is not always straightforward. Palladium-catalyzed direct arylation of heteroaryl derivatives with aryl halides has proven to be a very powerful method for the synthesis of a wide variety of arylated heteroarenes. However, palladium-catalyzed direct arylation of polyhalopyridines had not been investigated.
Henri Doucet and co-workers, University of Rennes, France, studied the conditions for the sequential direct monoarylation at C3, C4, or C5, of bromo-2-chloropyridines with a variety of heteroarenes, followed by arylation at the C2 position of the pyridyl ring by means of Suzuki coupling with use of a relatively low loading (1 mol %) of an air-stable palladium catalyst associated with a cheap and nontoxic base.

The results indicated that the commercially available catalyst was very effective, and this new procedure presents an improvement with respect to previous methods.
- Sequential Palladium-Catalysed Direct Arylation Followed by Suzuki Coupling of Bromo-2-chloropyridines: Simple Access to a Variety of 2-Arylpyridines,
Marya Baloch, David Roy, Souhila Bensaid, Véronique Guerchais, Henri Doucet,
Eur. J. Inorg. Chem. 2012.
DOI: 10.1002/ejic.201200613