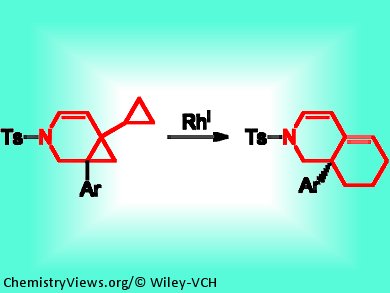
Using conventional synthetic pathways, arylhexahydroquinolines are difficult to prepare. Young Keun Chung and co-workers, Seoul National University, Korea, have developed a synthetic strategy from azabicyclo[4.1.0]heptene derivatives using Rh(PPh3)2(CO)Cl/AgBF4 in 1,2-dichloroethane. The bicyclo[4.1.0]heptene derivatives are easily accessible from the corresponding cyclopropylenynes via PtCl2-catalyzed cycloisomerization. By screening different derivatives of bicyclo[4.1.0]heptenes, it became apparent that an aryl group was needed in the 1-position and a cyclopropyl group in the 6-position for efficient rearrangement to take place.
A plausible reaction mechanism is proposed.
 Rhodium-Catalyzed Rearrangement Reaction of Azabicyclo[4.1.0]heptenes Bearing Cyclopropyl and Aryl Groups to Arylhexahydroisoquinolines,
Rhodium-Catalyzed Rearrangement Reaction of Azabicyclo[4.1.0]heptenes Bearing Cyclopropyl and Aryl Groups to Arylhexahydroisoquinolines,
Sori Son, Sun Young Kim, Young Keun Chung,
ChemistryOpen 2012, 1(4), 169–172.
DOI: 10.1002/open.201200022
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)