Electroactive biomolecules adsorb at the interface between two immiscible electrolyte solutions, where transfer of electrolyte anions is facilitated and can be detected voltammetrically. Typically, this interface is between an aqueous and an organic solution, each with a dissolved electrolyte.
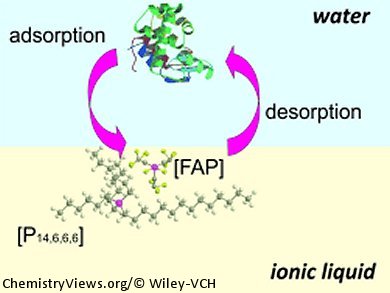
A similar setup for probing electrochemical activity at a water/room-temperature ionic liquid interface has been developed by Damien Arrigan and co-workers, Curtin University, Perth, Australia. They used a silicon membrane with micromachined pores to create a microinterface array for detection of hen egg white lysozyme.
When the lysozyme was dissolved in neutral water, they were not able to detect interfacial charge transfer. When the water phase was acidified with HCl to pH 2, however, the lysozyme was fully protonated with 17 positive charges. When a constant potential of 0.1 V was applied, the protein adorbed at the interface. It could then be detected using a desorptive voltammetric scan. During this scan, the anion of the ionic liquid was transferred across the interfacial boundary, and the charge transfer was detected as current. The magnitude of the desorption peak current varied linearly with the aqueous-phase concentration of the protein, which could be detected down to a concentration of 2.53 mm.
- Behavior of Lysozyme at the Electrified Water/Room Temperature Ionic Liquid Interface,
Eva Alvarez de Eulate, Debbie S. Silvester, Damien W. M. Arrigan,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200390