Timed Release of Orally Available Drugs
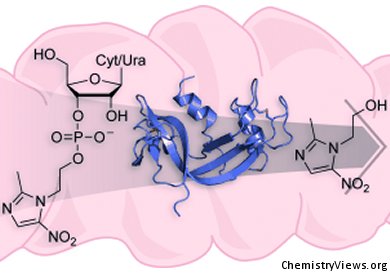
Many drug candidates fail because of low solubility or poor pharmacokinetic behavior. Ron Raines and colleagues at the University of Wisconsin–Madison, USA, have devised a new prodrug strategy to overcome these limitations. Their approach takes advantage of the capacity of an enzyme prevalent in human serum–pancreatic-type ribonuclease—to catalyze the cleavage of a drug conjugated to a ribonucleoside 3′-phosphate; the results of their work are reported in ChemMedChem.
They demonstrated efficacy by using metronidazole, a sparingly soluble antibiotic that is often administered orally. In the absence of ribonuclease, metronidazole conjugates are stable and inactive. Physiological levels of enzyme render the conjugate toxic to Bacteroides fragilis, a common penicillin-resistant bacillus that is responsible for anaerobic infections. Alterations to the ribonucleoside enable modulation of key attributes, such as the rate of drug delivery. This study paves the way for further research into the activation and timed release of drugs of varying aqueous solubility.
- Ribonucleoside 3′-Phosphates as Pro-Moieties for an Orally Administered Drug,
Michael J. Palte, Amy K. F. Davis, Nicholas A. McGrath, Carol A. Spiegel, Ronald T. Raines,
ChemMedChem 2012, 7(8).
DOI: 10.1002/cmdc.201200243