Generating hydrogen from water is a topic of intense research. The generation of hydrogen by using solar energy is one of the most feasible processes, as solar energy is abundant and environmentally favorable.
The problem to be solved is to concentrate the energy of sunlight in such a way that it is capable of cleaving water molecules into hydrogen and oxygen. This is possible by using molecules that can absorb sunlight and transfer its energy to catalysts that can then break the bonds in a water molecule. Iridium complexes are known to have appropriate characteristics for transferring light energy to a catalyst. However, their light absorption ability, especially in the visible region of the spectrum, needs to be enhanced.
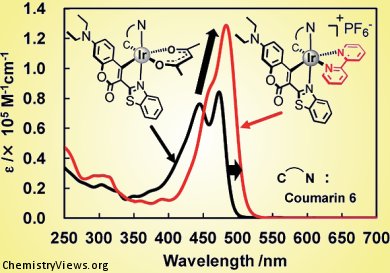
Shin-ya Takizawa, Shigeru Murata, and co-workers, University of Tokyo, Japan, prepared cationic iridium(III) complexes coordinated to two coumarin and one diimine ancillary ligand. These complexes showed significantly increased absorption of visible light by the synergic effect of the intraligand charge-transfer (ILCT) transition in the chromophoric coumarin moiety and the metal-ligand-to-ligand CT (MLLCT) and/or ligand-to-ligand CT (LLCT) transitions associated with the diimine ligand.
Specifically, the molar extinction coefficient of one of the complexes reached an unprecedentedly high value relative to those of other recently reported Ir-based dyes.
Image: © Wiley-VCH
- Cationic Iridium Complexes Coordinated with Coumarin Dyes – Sensitizers for Visible-Light-Driven Hydrogen Generation,
Shin-ya Takizawa, César Pérez-Bolívar, Pavel Anzenbacher Jr., Shigeru Murata,
Eur. J. Inorg. Chem. 2012.
DOI: 10.1002/ejic.201200474




Was the Hydrogen formed here stable..?? and at which wavelength actually did this happened..??