Carbon Dioxide Conversion to Methanol
Fossil-based resources are declining and their use releases the greenhouse gas CO2. Both of these problems could be significantly mitigated if we could use CO2 as a carbon source for the production of fuels and chemical feedstocks. Various different approaches are currently being explored for the catalytic conversion of CO2 to methanol (CH3OH). In the journal Angewandte Chemie, German researchers have now introduced a new possibility to conduct this stepwise reaction of CO2 in solution with help of a homogeneous catalyst.
Methanol and its products can not only be used as a fuel or for driving fuel cells, they are also a versatile feedstock for chemical industry. The conventional industrial process for the production of methanol starts with syngas, a mixture of hydrogen and carbon monoxide obtained from fossil resources. The process requires extremely high pressures and temperatures, involving a heterogeneous catalyst, which is a solid and therefore in a different phase than the gaseous or liquid educts and products.
A number of approaches for converting carbon dioxide (CO2) to methanol (CH3OH) have been developed. The big challenge for catalytic researchers is not only to activate the very stable CO2 molecule but also to catalyze the multistep conversion to methanol. Tailored catalysts are the key to enable the activation of this poorly reactive C1 building block.
First Homogenous Hydrogenation of CO2 to Methanol
Scientists from the RWTH Aachen have pursued a new approach to obtain methanol by the hydrogenation of CO2 with elemental hydrogen. While most previous methods use heterogeneous catalysts, this process is homogeneous. This means that the catalyst and the reactants are in the same phase, a solution. Homogeneous catalysis often require milder reaction conditions and the targeted development of the catalyst often enables a better selectivity. However, a homogeneous metal complex that is able to catalyze the multistep conversion of CO2 and hydrogen into methanol has not yet been reported.
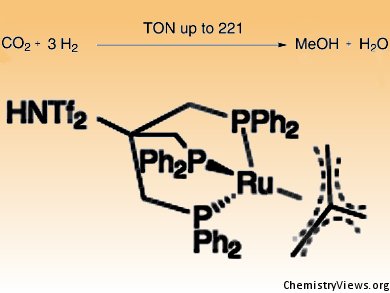
The team led by Jürgen Klankermayer and Walter Leitner has now developed a tailored catalyst for this complex conversion, namely a special ruthenium phosphine complex. The catalyst is dissolved in a solvent, in the simplest case in methanol itself, and put under pressure together with CO2 and hydrogen in an autoclave. It subsequently connects a molecule of CO2 in a stepwise fashion with three molecules of hydrogen to produce methanol and water.
“This is the first example of the hydrogenation of CO2 to methanol by use of a molecularly defined catalyst under relatively mild reaction conditions,” explain Leitner and Klankermayer. “We are now investigating in detail how the reaction works in order to develop our catalyst further.”
Image: © Wiley-VCH
- Hydrogenation of Carbon Dioxide to Methanol by Using a Homogeneous Ruthenium–Phosphine Catalyst,
Sebastian Wesselbaum, Thorsten vom Stein, Jürgen Klankermayer, Walter Leitner,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201202320




Please, read the article of the Carbon Insight about this topic. This process is going in water solution, atmospheric pressure, room temperature, without outer energy and catalyst and produce carbon from carbon dioxide. Dr Endre Simonyi