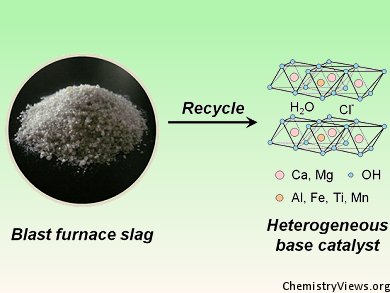
The preparation of catalysts involves sophisticated procedures, and many hours are spent in the lab before an optimal catalyst formulation is found. In heterogeneous catalysis, the difficulty often is to achieve a stable dispersion of metal particles on a support surface or the tuning of a solid catalyst’s acid/base properties to the desired level by doping.
Hiromi Yamashita and co-workers, University of Osaka, Japan, report a more pragmatic approach to the latter problem: realizing that the material was potentially rich in metals, they collected slag from an iron blast furnace and converted it into a layered double hydroxide (LDH) by simple dissolution and precipitation. Chemical analysis of the LDH samples showed them to be hydrocalumites, with magnesium, iron, titanium, and manganese cations substituting at either calcium or aluminum sites.
The slag-derived metals in the hydrocalumites functioned as either active sites or promoters for several chemical reactions. With performances comparable to, or better than, traditional catalysts, a waste product was thus turned into a chemical workhorse. Contact your local blast furnace for more information!
Image: © Wiley-VCH
- Waste-Slag Hydrocalumite and Derivatives as Heterogeneous Base Catalysts,
Y. Kuwahara, K. Tsuji, T. Ohmichi, T. Kamegawa, K. Mori, H. Yamashita,
ChemSusChem 2012, 5.
DOI: 10.1002/cssc.201100814