The Morita-Baylis-Hillman (MBH) reaction, an atom-economical carbon-carbon bond forming reaction, has great potential in organic synthesis. It delivers an array of densely functionalized α-methylene-β-hydroxy derivatives from an aldehyde and an α,β-unsaturated electron-withdrawing group in the presence of a nucleophilic catalyst.
The reaction is typically slow, however, Masahiro Terada and co-workers, Tohoku University, Japan, found a guanidine-azole binary system to catalyze the MBH reaction of aldehydes with cyclic enones within 24 h at room temperature with product yields of >98 %.
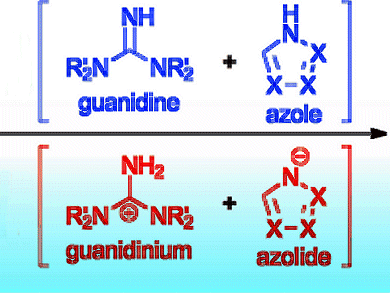
Ion pairs form between the intermediates of the guanidinium and azolide derivatives (pictured), which have strong basicity and a proton transfer function, respectively. The formation of hydrogen and covalent bonds between the catalyst and reaction intermediates stabilize the reaction system. This route, thus, sidesteps the unstable zwitterionic intermediates that are known to hinder the MBH reaction.

Further investigation showed axially chiral guanidines co-catalysts to foster a new stereogenic center (72 % ee). The reaction of the aldehyde and enolate intermediate is key here and further mechanistic details are under investigation.
- Guanidine/Azole Binary System as an Efficient Catalyst for Morita–Baylis–Hillman Reaction,
Masahiro Terada, Satoko Fukuchi, Kei Amagai, Megumi Nakano, Hitoshi Ube,
ChemCatChem 2012, 4, 963–967.
DOI: 10.1002/cctc.201200149