Bis-alkylamidines compounds, bioisosteres of bis-thiazolium salts, are potent antimalaric drugs developed to fight multidrug resistant strains of the parasite Plasmodium. These drugs interfere efficiently with the parasite’s phosphatidylcholine biosynthesis by acting as choline analogs. But they can be administered only parenterally as their cationic charges prevent a good oral bioavailability.
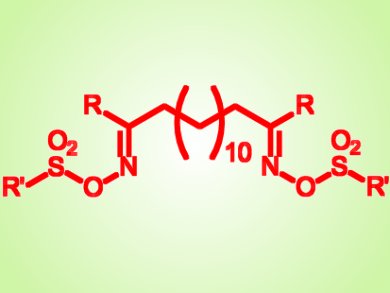
In order to circumvent this problem and mask the charges, Mélissa Degardin and colleagues, Université de Montpellier, France, used a prodrug strategy consisting of sulfonate derivatives of bis-alkylamidoximes.
The team synthetized 25 new sulfonates and demonstrated that O-alkylsulfonate derivatives possess the best oral antimalaric activity. The alkyl substituent on the sulfonate group likely affects the lipophilicity of the compunds and their ability to diffuse through cellular membranes, two parameters influencing oral absorption.
Directly converted into their corresponding active alkylamidines, bis-alkylamidoxime O-alkylsulfonates could be therefore promising oral antimalaric drugs.
- Evaluation of bis-alkylamidoxime O-alkylsulfonates as orally available antimalarials,
M. Degardin, S. Wein, S. Gouni, C. Tran Van Ba, J. F. Duckert, T. Durand, R. Escale, H. Vial, Y. Vo-Hoang,
ChemMedChem 2012, 7(6), 991–1001.
DOI: 10.1002/cmdc.201200112