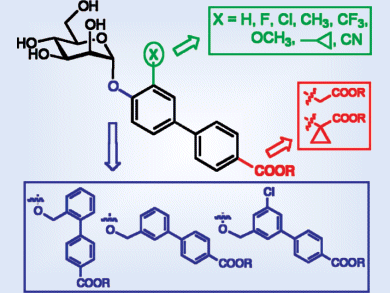
Urinary tract infections (UTIs), caused primarily by uropathogenic E. coli (UPEC), affect millions of people and account for significant morbidity and high medical costs. UPEC strains encode filamentous surface-adhesive organelles called type 1 pili. FimH is located at the tips of these pili. The initial attachment of UPEC to host cells is mediated by interactions between FimH and oligomannosides on urothelial cells. Blocking these lectins with carbohydrates or analogues thereof prevents bacterial adhesion to host cells, offering a potential way to prevent and treat UTIs.
Beat Ernst and colleagues, University of Basel, Switzerland, synthesized a series of biphenyl α-D-mannosides as FimH antagonists with the aim of improving binding affinity and optimizing pharmacokinetic properties. Both structure–activity and structure–property relationships were established for these biphenyl α-D-mannosides. Correlation between van der Waals volumes of these substituents and enthalpy indicated the importance of shape complementary. This correlation of enthalpic improvements with van der Waals volume values will provide a useful tool for further structural optimization.
Image: © Wiley-VCH
- FimH Antagonists: Structure–Activity and Structure–Property Relationships for Biphenyl α-D-Mannopyranosides,
L. Pang, S. Kleeb, K. Lemme, S. Rabbani, M. Scharenberg, A. Zalewski, F. Schädler, O. Schwardt, B. Ernst,
ChemMedChem 2012, 7(08).
DOI: 10.1002/cmdc.201200125