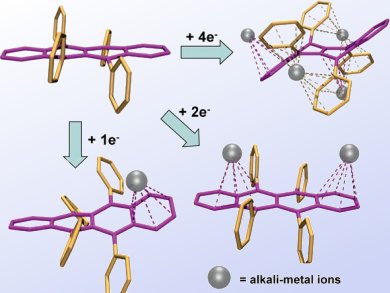
The shape and physical properties of materials determine their usefulness. Being able to change these attributes in a controlled way makes it possible to optimize a material for a specific application.
Marina Petrukhina and co-workers, University at Albany, State University of New York, USA, prepared mono-, di-, and tetraanions of rubrene (Rub) by a stepwise alkali-metal reduction. Rubrene is a nonpolar polyarene having multiple metal binding sites and sufficient skeleton flexibility. It is known to be an efficient material for the fabrication of organic field-effect transistors and light-emitting diodes.
The geometries of charged rubrene anions and their supramolecular aggregation with alkali-metal ions (pictured) in the solid state and solution are discussed based on single-crystal X-ray diffraction studies, ESR, and multinuclear NMR spectroscopy.
Image: © Wiley-VCH
- Reshaping Rubrene by Controlled Reduction with Alkali Metals,
A. V. Zabula, N. J. Sumner, A. S. Filatov, S. N. Spisak, V. M. Grigoryants, M. A. Petrukhina,
Eur. J. Inorg. Chem. 2012.
DOI: 10.1002/ejic.201200164