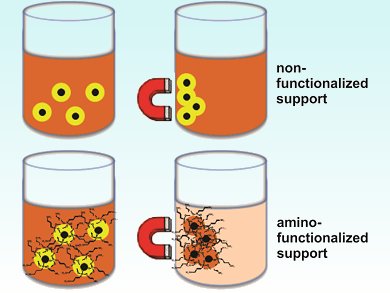
The immobilization of metal nanoparticles in magnetic responsive solids allows the easy, fast, and clean separation of catalysts. However, the efficiency of this separation process depends on a strong metal–support interaction. This interaction can be enhanced by functionalizing the support surface with amino groups.
For example, iridium nanoparticles supported on Fe3O4 encapsulated in silica act as an easily isolable hydrogenation catalyst. An unusually mild reduction of IrCl3 in an H2 atmosphere yields the Ir0 nanoparticles. The permanent magnet in the catalyst support allows for isolation of the catalyst without further chemical or physical procedures by application of a magnet to the reactor wall.
Liane Rossi and co-workers, Universidade de São Paulo, Brazil, tested this magnetic catalyst in the solvent-free hydrogenation of cyclohexene. The catalyst system gave >99 % conversion over six runs and an accumulated turnover frequency of 12600 molsubstrate molmetal–1 at mild temperature and pressure (75 °C, 6 atm H2).
Reaction of the catalyst with 3-aminopropytriethoxysilane provides an NH2-functionalized molecular silica layer. These amino-functionalized support captured iridium species show increased uptake of Ir3+ ions from solution compared with analogous Ir0 catalysts prepared with use of the nonfunctionalized support.
This modified surface enriched with NH2 groups is thought to affect the nanoparticle formation, stabilization, and catalytic performance. The Fe3O4@SiO2-NH2-Ir0 catalyst’s core-shell morphology was evidenced by transmission electron microscopy (TEM) analysis and the presence of Ir on the silica surface by using energy-dispersive X-ray spectroscopy.
Image: © Wiley-VCH
- Catalyst Recovery and Recycling Facilitated by Magnetic Separation: Iridium and Other Metal Nanoparticles,
M. J. Jacinto, F. P. Silva, P. K. Kiyohara, R. Landers, L. M. Rossi,
ChemCatChem 2012, 4, 698-703.
DOI: 10.1002/cctc.201100415