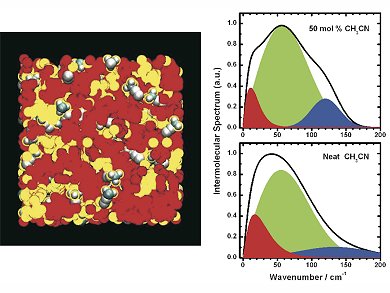
Ionic liquids are salts in the liquid state. Because of their chemical composition, they also possess oily properties, with the oil-like parts forming pools within the salt matrix.
Scientists led by Edward Quitevis, Texas Tech University, USA, and Gergory Voth, University of Chicago, USA, have combined ultrafast spectroscopic techniques with computer simulations to study the behavior of ionic liquids at the atomic level on very short timescales. With the help of computer simulations, the team could show that the molecules of the solvent acetonitrile are usually found at the oil-salt boundaries of ionic liquids. They also proved this experimentally by using lasers that produce very short pulses of light, only tens of femtoseconds in duration.
The team say that because of the way the salt and oil regions are arranged in the ionic liquids – and the nanometer size of these regions – ionic liquids could be used as “two-in-one nanoreactors” for chemical reactions, with the reactants confined in the regions of the liquid where they tend to want to be.
Image: © Wiley-VCH
- Nanostructural Organization in Acetonitrile/Ionic Liquid Mixtures: Molecular Dynamics Simulations and Optical Kerr Effect Spectroscopy,
F. Bardak, D. Xiao, L. G. Hines Jr., P. Son, R. A. Bartsch, E. L. Quitevis, P. Yang, G. A. Voth,
ChemPhysChem, 2012, 13, 1687–1700.
DOI: 10.1002/cphc.201200026