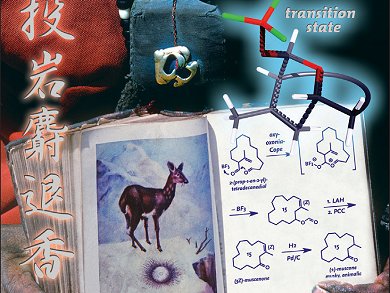
Musk odorants are indispensable in perfumery. The two most important musk families at present are macrocycles, derived from the natural lead muscone, and linear alicyclic musks, such as 1”'(2”’)-dehydro-Serenolide. The odor of these has been attributed to horseshoeshaped conformers that mimic macrocyclic rings on the odorant receptors. The synthesis of further unsaturated macrocyclic musks can shed light upon similarities in the structure–odor correlation of these two musk families.
Quanrui Wang, Fudan University, Shanghai, China, Philip Kraft, Givaudan Schweiz AG, Dübendorf, Switzerland, and colleagues report on a new oxyoxonia-Cope macrocyclization to (3Z)-configured cycloalk-3-en-1-yl formats.
These findings proved useful in the synthesis of several unsaturated and saturated macrocyclic ketones, including (±)-muscone (see scheme). The synthesized structures provide new insight into the structure–odor correlation of musks, resulting in an olfactophore model, which makes it likely that macrocyclic and linear alicyclic musks address the same set of olfactory receptors.
- Efficient Macrocyclization by a Novel Oxy-Oxonia-Cope Reaction: Synthesis and Olfactory Properties of New Macrocyclic Musks,
Yue Zou, Halima Mouhib, Wolfgang Stahl, Andreas Goeke, Quanrui Wang, Philip Kraft,
Chem. Eur. J. 2012, 23.
DOI: 10.1002/chem.201200882