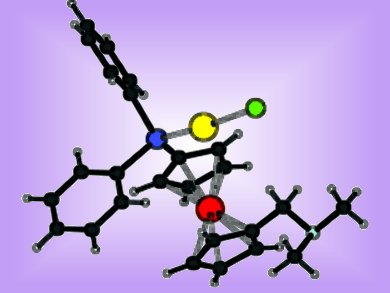
Petr Štěpnička and co-workers, Charles University, Prague, Czech Republic, describe a convenient alternative synthetic route to a dimethylaminomethyl-functionalized phosphanylferrocene ligand, avoiding the use of toxic starting materials. The complexation of this hybrid P,N-donor was studied with tetrahydrothiophene (tht)-containing [AuCl(tht)] and cycloocta-1,5-diene (cod)-containing [PdCl2(cod)].
Single-crystal X-ray diffraction analysis revealed the formation of an expected phosphane–Au complex when treated with [AuCl(tht)]. Traces of hydrogen chloride formed by gradual decomposition of the complex in solution, causes a protonation of the amine group within the complex.
With [PdCl2(cod)] coordination of both the P and N donors of the ligand was expected. In fact, the complex formation was dependent on the ratio of ligand to metal. While a 1:1 ratio afforded a bis(phosphane) complex, a 2:1 ratio led to the formation of a ligand-bridged dimer. A subsequent reaction of the dimer with traces of hydrogen chloride again caused a protonation of the amine group leading to a monomeric zwitterionic complex.
Image: © Wiley-VCH
- An Alternative Preparation of 1-(N,N-Dimethylaminomethyl)-1′-(diphenylphosphanyl)ferrocene: Synthesis and Structural Characterization of AuI and PdII Complexes with this Hybrid Ligand,
P. Štěpnička, M. Zábranský, I. Císařová,
ChemistryOpen 2012, 1(2), 71–79.
DOI: 10.1002/open.201200004
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.