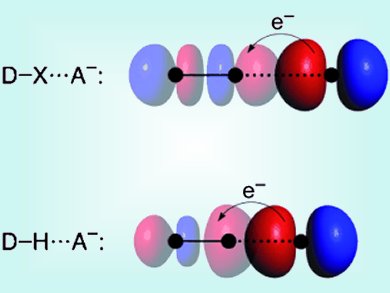
The unique features of water and DNA’s structure are only two important examples in which hydrogen bonds play crucial roles. Hydrogen bonds have therefore been intensively studied. Very similar in nature to hydrogen bonds (DH⋅⋅⋅A−) are halogen bonds (DX⋅⋅⋅A−; D, X, A = F, Cl, Br, I). Both have a sizeable covalent component in addition to their electrostatic interaction.
Based on quantitative Kohn–Sham molecular orbital (MO) theory, energy decomposition analyses (EDA) and Voronoi deformation density (VDD) analyses of the charge distribution, Lando Wolters and Matthias Bickelhaupt, Vrije University Amsterdam, The Netherlands, present an accurate physical model of the halogen bond. Thereby the authors want to create a set of consistent structural and energy data for the reliable calculation of trends over a wide range of model systems. They present a detailed understanding of the nature of halogen bonds and of the similarities and differences to the better understood hydrogen bonds. They find that the halogen bond in DX⋅⋅⋅A− arises not only from classical electrostatic attraction, but also receives substantial stabilization from HOMO–LUMO interactions between the lone pair of A− and the σ* orbital of D–X.
 Halogen Bonding versus Hydrogen Bonding: A Molecular Orbital Perspective,
Halogen Bonding versus Hydrogen Bonding: A Molecular Orbital Perspective,
L. P. Wolters, F. M. Bickelhaupt,
ChemistryOpen 2012, 1(2), 96–105.
DOI: 10.1002/open.201100015
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.




This paper is an excellent documento for academic purposes in many topics of general chemistry and environmental chemistry applications. M.Sc. Julio C. Bracho