Domino Route to Anticonvulsants and Others
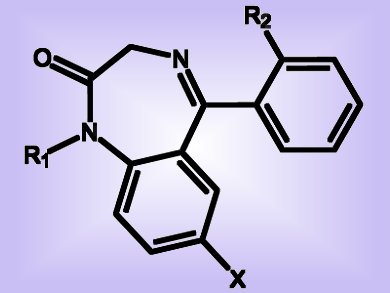
Drugs such as sedatives, hypnotics, and anticonvulsants often contain a benzodiazepine moiety (pictured). They are useful in the treatment of, for example, anxiety, insomnia, agitation, and seizures. The 1,5-benzodiazepin-2-one skeleton in particular is considered a “privileged” scaffold in medicinal chemistry and drug development. The pharmacological importance of benzodiazepine derivatives therefore justifies new efforts for their synthesis. As recently reported in the European Journal of Organic Chemistry, a Columbian/French team led by Yoann Coquerel and Jean Rodriguez have now introduced a general domino sequence to provide new synthetic entry to 1,3-dihydro-2H-1,5-benzodiazepin-2-ones.
An important focus of modern organic synthesis is economy, and many transformations have been developed to allow the creation of molecular complexity and functional diversity in a single chemical operation. These transformations are best performed with polyfunctionalized substrates in which the distinct reactive groups react selectively in a well-defined order to give a single product. α-Oxo ketenes exhibiting two carbonyl groups and one double bond distributed over three carbon atoms are highly reactive molecules that fall into this class of compounds. α-Oxo ketenes have already been used to prepare a number of heterocycles, and the scientists have already shown that these compounds can be obtained through the rearrangement of 2-diazo-1,3-dicarbonyl compounds. The team was further interested in the reactions of 2-diazoalkane-1,3-diones with bis(N-nucleophiles) in a domino sequence to prepare the desired benzodiazepine derivatives.
Microwave-Assisted Domino Benzannulation
Although synthetic routes to benzodiazepines exist, they are often sensitive to the type of substrate and the reaction conditions; moreover, these methods usually require long reaction times and are often complicated by side reactions. Importantly, using their domino methodology and without any additives, the researchers found that microwave irradiation of a 1:1 mixture of a set of diazo compounds with o-phenylenediamine furnished a series of bi- and tricyclic 1,3-dihydro-2H-1,5-benzodiazepin-2-ones, which are among the most promising compounds containing the benzodiazepine structure. The authors postulate that the reaction follows this sequence: rearrangement, nucleophilic addition, and intramolecular imination. This reaction represents a new and advantageously complementary synthetic approach to a valuable class of drug-like compounds.
- Microwave-Assisted Domino Benzannulation of α-Oxo Ketenes: Preparation of 1,3-Dihydro-2H-1,5-benzodiazepin-2-ones,
J.-C. Castillo, M. Presset, R. Abonia, Y. Coquerel, J. Rodriguez,
Eur. J. Org. Chem. 2012.
DOI: 10.1002/ejoc.201200093