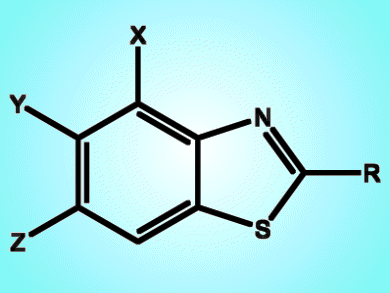
The synthesis of 2-substituted azoles has attracted much attention because of their important roles in bioactive pharmaceuticals and new materials. In particular, benzothiazoles have been intensively studied. They can be used in biological applications, such as antitumor, anti-inflammatory, antimicrobial, antitrypanosomal, anticonvulsant, and antituberculosis agents, and they can also be used as tracers for β-amyloid plaques in Alzheimer’s disease. Benzothiazole moieties are also found in functional molecules such as nonlinear optical materials and ligands for phosphorescent complexes. Much effort has therefore been devoted to the development of new methodologies for the construction of this privileged structure.
Sunwoo Lee and Kwang Ho Song, Chonnam National University and Korea University, respectively, both in Republic of Korea, have reported a new protocol to access benzothiazoles by using sodium hydrosulfide as a sulfur surrogate through a copper-catalyzed three-component reaction. The procedure is robust and operates at extremely low loadings of copper. This methodology is important, as sulfur-containing nucleophiles usually poison the catalyst in cross-coupling reactions. Use of a sulfur surrogate thus provides efficient access to benzothiazoles as potentially interesting biological materials.
- Synthesis of Benzothiazoles through Copper-Catalyzed One-Pot Three-Component Reactions with Use of Sodium Hydrosulfide as a Sulfur Surrogate,
N. Park, Y. Heo, M. R. Kumar, Y. Kim, K. H. Song, S. Lee,
Eur. J. Org. Chem. 2012.
DOI: 10.1002/ejoc.201101773