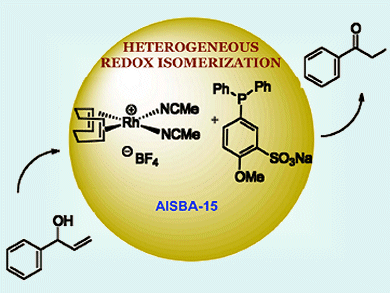
Catalytic isomerization of allylic alcohols to carbonyl compounds is a simple, atom-economical, and valuable transformation. High yields in this reaction can achieved by homogeneous transition metal catalysis, however, separation of the products from the metal complex can be difficult. Xiaodong Zou, Belén Martín-Matute and co-workers, Stockholm University, Sweden, have developed a heterogeneous catalysis by anchoring cationic rhodium(I) complexes on inorganic mesoporous supports, making for a simple product separation.
Rh+ with sulfonated phosphine ligands immobilized on the mesoporous AlSBA-15 material performed the best out of the catalyst−support systems tested. Eleven different allylic alcohols were selectively isomerized with yields as high as >95 %, turnover numbers of up to 198 molproduct molcatalyst–1, and a minimum fivefold recyclability. Catalyst loadings were as low as 0.5 mol %.
Aliphatic primary allylic alcohols, typically difficult to isomerize, and those with a disubstituted double bond were also transformed into carbonyl compounds. Characterization results confirmed the integrity of the immobilization. Alongside hydrogen bonding and ionic interactions, the robustness of the system indicates that covalent bonding may also be present.
- Single Site Supported Cationic Rhodium(I) Complexes for the Selective Redox Isomerization of Allylic Alcohols,
S. Sahoo, H. Lundberg, M. Edén, N. Ahlsten, W. Wan, X. Zou, B. Martín-Matute,
ChemCatChem 2012, 4, 243–250.
DOI:10.1002/cctc.201100321