The design of low molecular weight gelators is a great challenge. New gelators are dependent on the subtle interplay between the relative solubility of the molecule and key structural features such as hydrogen bonding receptors and aromatic stacking units plus environmental factors such as temperature or pH.
Wayne Hayes, University of Reading, UK, and colleagues, synthesized and characterized a focused library of potential low molecular weight hydrogelators. Each contains two substituted aromatic residues separated by a urea or thiourea linkage. The rational synthesis of this library has resulted in the production of a series of structurally simple and easily accessible supergelators.
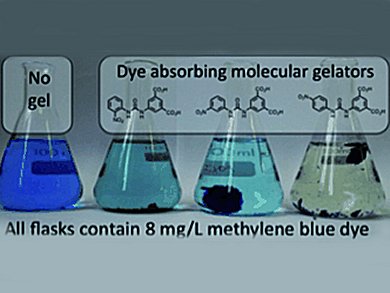
Gels were formed through a pH switching methodology, by acidification of a basic solution either by addition of HCl or via the slow hydrolysis of glucono-δ-lactone. The resulting pH switchable, colour changing gels can sequester large, but varying quantities of the aromatic cationic dye, methylene blue, from aqueous solution (up to 1.02 g of dye per gram of dry gelator).
Cryo-transmission electron microscopy of two of the gels revealed an extensive network of high aspect ratio fibers. The structure of the fibers altered dramatically upon addition of 20 wt % of the dye, resulting in aggregation and significant shortening of the fibrils.
The structure/property relationships revealed by this study will guide the production of novel inexpensive and easy to use water purification platforms.
- pH Tunable Hydrogelators for Water Purification: Structural Optimisation and Evaluation,
Daniel M. Wood, Barnaby W. Greenland, Aaron L. Acton, Francisco Rodríguez-Llansola, Claire A. Murray, Christine J. Cardin, Juan F. Miravet, Beatriu Escuder, Ian W. Hamley, Wayne Hayes,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201102137 - Movie demonstrating the dramatic dye uptake by the hydrogelator