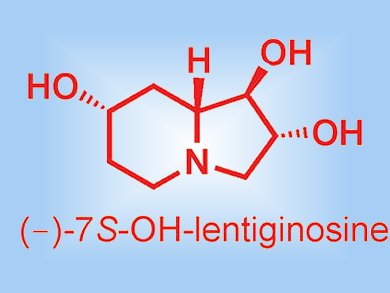
Useful improvements have been achieved for the synthesis of a cyclic nitrone derived from tartaric acid. The nitrone was subsequently used for the synthesis of the iminosugar (–)-7S-OH-lentiginosine. Franca Cordero and co-workers, University of Florence, Italy, found that the key intermediate, 3,4-bis-tert-butoxypyrroline N-oxide (see scheme), was obtained in much better yield by treatment with tert-butyl acetate in presence of HClO4 than the current best method involving a troublesome etherification of two vicinal hydroxy groups.

Like the recently discovered natural indolizidine iminosugar, (+)-lentiginosine, the synthesized iminosugar shows interesting proapoptotic activity against cancer cells, but low cytotoxicity towards normal cells. This was shown by testing against cell lines of different origin. The authors suggest that these data demonstrate how these compounds, with a structure resembling that of sugar mimetic glycosidase inhibitors, endowed with previously unknown pro-apoptotic activity, could constitute a new distinct class of pro-apoptotic agents.
- (–)-(1R,2R,7S,8aR)-1,2,7-Trihydroxyindolizidine ((–)-7S-OH-Lentiginosine): Synthesis and Proapoptotic Activity,
F. M. Cordero, P. Bonanno, B. B. Khairnar, F. Cardona, A. Brandi, B. Macchi, A. Minutolo, S. Grelli, A. Mastino,
ChemPlusChem 2012.
DOI: 10.1002/cplu.201100069
This article is available for free as part of the ChemPlusChem free trial.